What Is Oxidized in a Galvanic Cell Apex
The silver metal B. The nickel ions D.
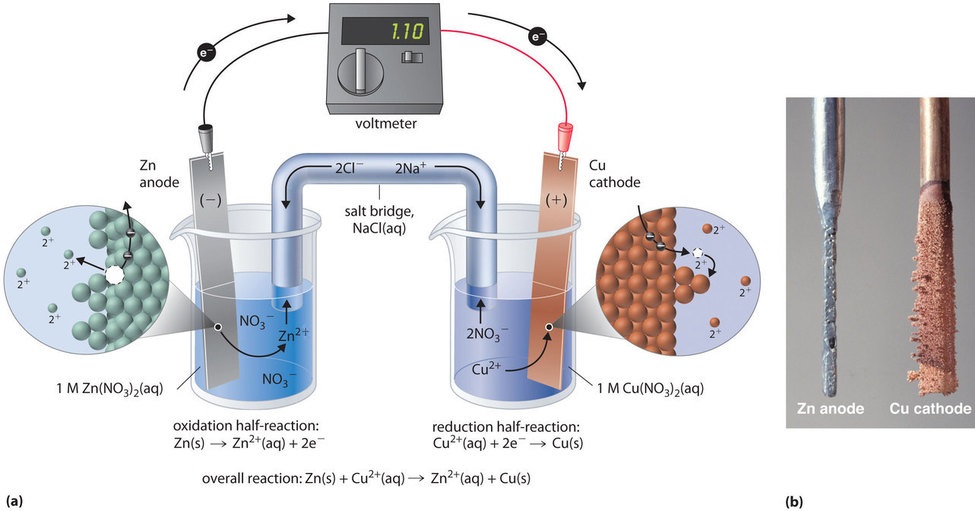
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts
The name refers to the flow of anions in the salt bridge toward it.

. E cell 08 025 105V. 1 point Mg - Mg 2 2 e- 2 Au 2 e- - 2 Au. What is oxidized in a galvanic cell made with silver and nickel electrodes.
So to calculate E cell subtract the least ve E from the most ve E value. Left to right in the standard galvanic cell in the figure. Add your answer and earn points.
What is the total reduction potential of a cell in which Lithium Li is reduced and mercury Hg is oxidized. Multiply each half-reaction by the correct number in order to balance charges for the two half-reactions. If one electrode in a galvanic cell is made of zinc Zn and one is made of silver Ag which metal would be the cathode and which would be the anode.
Electrons flow from the anode to the cathode. The net cell reaction in a particular galvanic cell is. The second is therefore forms the cathode compartment.
Cu2aq Sn2aq Cu s Sn4aq In this cell we would expect to observe. A galvanic cell is a chemical reaction that produces an electrical current and redox reactions are what transpires in galvanic cells. In a galvanic cell the component with lower standard reduction potential gets oxidized and that it is added to the anode compartment.
The silver ions 1 See answer Advertisement Advertisement zachjames2005 is waiting for your help. Know more differences between galvanic cells and electrolytic cells by visting us. A galvanic cell produces electrical energy through the conversion of chemical energy whereas the electrolytic cell carries out the conversion of the electrical energycurrent supplied to it into chemical energy.
Up to 24 cash back There is a lot going on in Figure 2 so it is useful to summarize things for this system. Mg - Mg2 2 e- 2 Au 2 e- - 2 Au. Mg - mg2 2 e- Reduction.
Crystals of copper forming on the anode. A galvanic cell generates current and an electrolytic cell uses electrical energy to cause redox reactions to occur. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through the conductor whereas in an electrolytic cell the redox reactions are influenced by an external source of current.
The solution in the copper half-cell becoming lighter in color. -390 V What is true of electrolytic cells but is not true of galvanic cells. Since almuninum has the lowest standard reduction potential xi-166V therefore it should be oxidized as follows.
Au e - - Au. The Ni2Ni 12 cell will therefore take in these electrons and move right to left. The cathode corroding away.
The nickel metal C. The voltage of the cell is an empirically measured quantity so must always have a ve value. What is oxidized in galvanic cell made with silver and nickel electrodes.
The electrode in the left half-cell is the anode because oxidation occurs here.
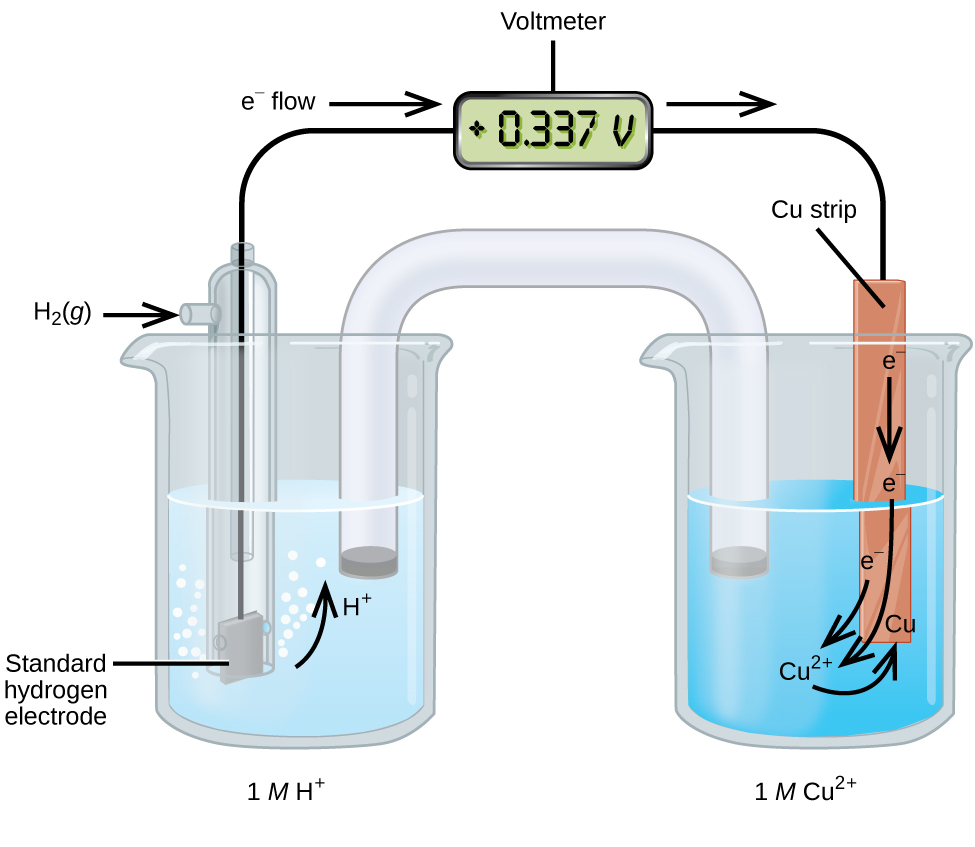
17 3 Standard Reduction Potentials Chemistry
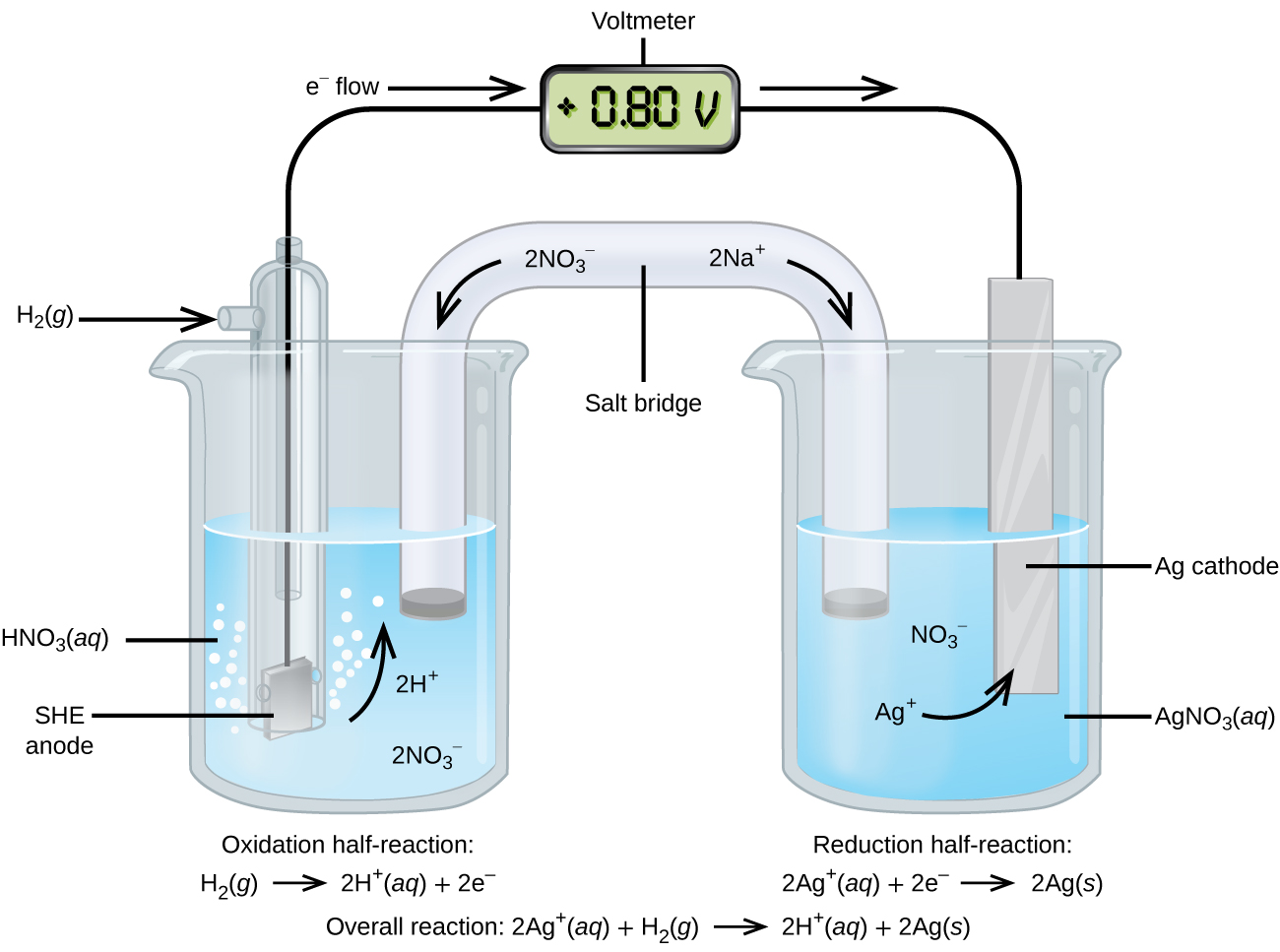
17 3 Standard Reduction Potentials Chemistry
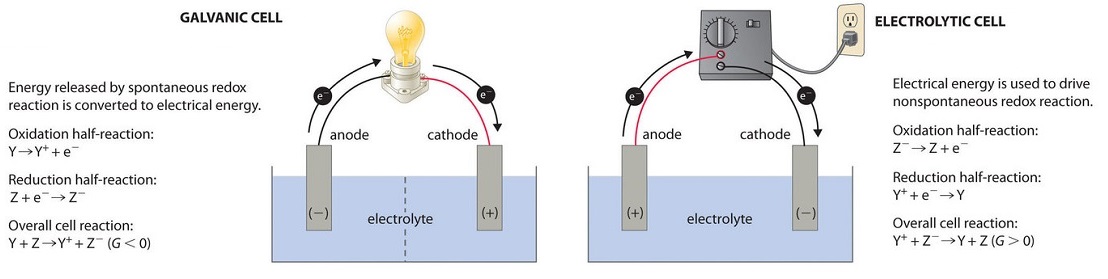
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts
Comments
Post a Comment